- THE TOP 10 SURGICAL ADVANCES OF THE LAST 70 YEARS - 30 Apr 2024
- BRAIN TRANSPLANTATION? - 28 Mar 2024
- THE ANESTHESIA CONSULTANT NAMED THE #1 ANESTHESIOLOGY BLOG IN THE WORLD FOR 2024 BY FEEDSPOT - 7 Mar 2024
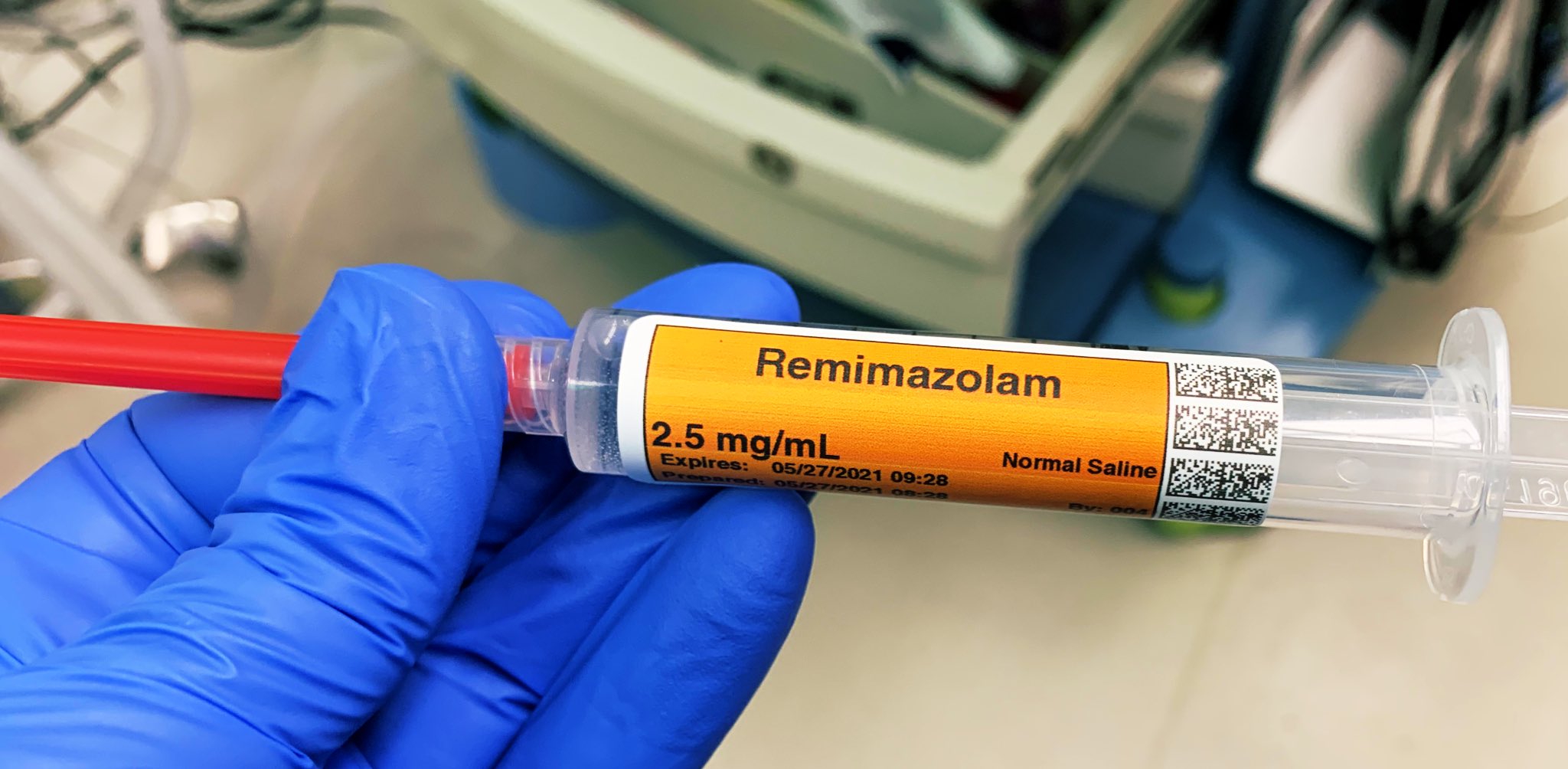
In July 2020 the Food and Drug Administration (FDA) approved the intravenous benzodiazepine remimazolam (Byfavo, Acacia Pharma) for use in sedation for procedures of 30 minutes or less. Will anyone utilize this new drug, or is it an expensive addition to our arsenal with few significant advantages over current agents?

Remimazolam differs from midazolam (Versed), the current most commonly used IV benzodiazepine, in that remimazolam is rapidly converted to an inactive metabolite by tissue esterases, resulting in an ultra-short onset/offset profile. Remimazolam is marketed as a powder which must be reconstituted into a liquid within its vial prior to administration.

For use in procedural sedation, remimazolam will not replace Versed, but rather will aim to replace propofol. The proposed advantages of remimazolam over propofol include:
- Remimazolam can be completely reversed by the benzodiazepine antagonist flumazenil (Romazicon) whereas there is no reversal agent or antagonist for propofol. The only way to end the sedative effects of propofol is for an anesthesia professional to support the airway, breathing, and circulation of the patient until the drug effects of propofol wear off in time.
- Remimazolam has minimal cardiac or respiratory depression. Sicker ASA III and IV patients maintain their breathing and circulation status while under remimazolam sedation.
- There is no accumulative effect of remimazolam over time. Its elimination by an esterase does not slow during lengthy administration of remimazolam, as in the prolonged sedation of an intensive care unit (ICU) patient on a ventilator.
- There is no burning sensation upon injecting remimazolam into a patient’s intravenous line as there is with propofol.
- A non-anesthesia-professional can administer remimazolam, whereas an anesthesia professional/airway expert must administer and monitor propofol administration.
Are these advantages important? Items 1 – 5 are discussed as follows:
- Non-anesthesiologists can reverse the effects of remimazolam with flumazenil if they overdose a patient, but this advantage is less important for anesthesia professionals. Anesthesiologists can manage the airway of a patient over-sedated with a benzodiazepine without need to administer a reversal agent. I’ve never administered a dose of flumazenil in my entire career, nor have most of my anesthesia colleagues.
- Propofol has cardiac and respiratory depression, but in most cases these effects are minimal. Per the PDR (Physician’s Digital Reference), patients with compromised myocardial function, intravascular volume depletion, or abnormally low vascular tone (e.g. septic patients) are more susceptible to hypotension. When an anesthesiologist is present these risks are routinely managed.
- For a long operating room anesthesia case (e.g. of 8 – 10 hours duration), there is no clinically significant accumulation of propofol in the bloodstream. Propofol Infusion Syndrome (PRIS), which can be potentially fatal, is a risk with prolonged propofol sedation in the ICU (See ICU Sedation below).
- The burning sensation upon injecting propofol can be blunted by intravenous lidocaine. A 2016 meta-analysis showed that both lidocaine pretreatment and mixing lidocaine with the propofol were effective in reducing pain on propofol injection. In addition, a preanesthetic dose of Versed prevents a patient from remembering any burning sensation from a propofol injection that follows.
- The most important advantage of remimazolam is that non-anesthesiologists can safely administer remimazolam. Propofol administration requires an experienced clinician, e.g. either an anesthesiologist, a certified registered nurse anesthetist (CRNA), or an emergency medicine physician. Per the American Society of Anesthesiologists: “The practitioner administering propofol for sedation/anesthesia should, at a minimum, have the education and training to identify and manage the airway and cardiovascular changes which occur in a patient who enters a state of general anesthesia.”
The disadvantages of remimazolam compared to propofol include:
- Expense. The cost of a 20 ml (200 mg) vial of propofol is $9.20. The cost of a 20 mg vial of powdered remimazolam is $41.67.
- Remimazolam is sold as a powder and must be reconstituted into a liquid before it can be injected intravenously.
Remimazolam is currently approved as an anesthesia drug in Japan and South Korea, for intensive care unit sedation in Belgium, but only for procedural sedation in the United States, China, and Europe. In total, there are four possible applications for remimazolam. Let’s examine the pros and cons of using remimazolam in these four applications:
- Preoperative sedation. Since midazolam (Versed) was approved in 1982, a standard anesthesia practice has included a 2 mg dose of Versed prior to surgery to calm a patient’s anxiety. In the 1980s my anesthesia chairman at Stanford received a letter from a postoperative patient in which she complained of being awake and very anxious in the operating room prior to the anesthetic for her breast cancer surgery. Our chairman lectured to us, “Do you know many patients are nervous prior to their anesthesia and surgery? Every one of them. We have an excellent drug for relieving preoperative anxiety, and that drug is Versed. Use it! Give your patient a dose of Versed before they enter the operating room. There are few significant side effects of one dose of Versed. Use it!” Will remimazolam replace Versed for this application? No. There is no advantage of the new, shorter acting, more expensive remimazolam over Versed for preoperative sedation.
- Sedation for short procedures. This is the FDA-approved application for remimazolam in the United States. An example procedure would be a colonoscopy. Will remimazolam be widely used for colonoscopies in the near future? No, I doubt it. The cost increase is the main disadvantage. See the typical drug acquisition costs for three alternative sedation recipes for colonoscopy below:
$18.40 for 400 mg of propofol; or
$5.17 for fentanyl+Versed ($4.35 dollars for 6 mg of Versed plus $0.82 for 200 micrograms of fentanyl); or
$41.67 for 20 mg of remimazolam.
The increased cost per case is $23.27 for remimazolam over propofol.
The increased cost per case is $36.50 for remimazolam over fentanyl+Versed.
If a busy endoscopy center does 100 colonoscopies cases per week, the cost increase is $2327 per week for remimazolam over propofol, or $3650 per week for remimazolam over fentanyl+Versed. These are a prohibitive cost increases with no clear added benefits. The only way remimazolam could result in cheaper sedation costs would be if a healthcare system was looking to eliminating the cost of paying for an anesthesia provider for these procedures. The pairing of remimazolam+gastroenterologist sedation rather than propofol+anesthesiologist sedation could afford significant cost savings for a healthcare system.
3. Total intravenous anesthesia (TIVA). TIVA could include a continuous infusion of the ultra-short-acting narcotic remifentanil plus a continuous infusion of the ultra-short-acting remimazolam. An alleged advantage of this technique could be the fast offset time of these two TIVA anesthetic agents. I doubt this technique will gain market share. It’s far easier to turn on the knob of a sevoflurane vaporizer than to load and manage two TIVA-syringe pumps. As well, the added expense of a prolonged infusion of remimazolam will be prohibitive.
4. ICU sedation. Remimazolam has the advantage of ongoing first-degree elimination, meaning that no matter how long the drug is infused, it will always have reliable elimination by esterase and will not accumulate in the plasma. Prolonged ICU sedation with propofol can lead to the Propofol Infusion Syndrome (PRIS). PRIS occurs predominantly in patients receiving high doses of propofol for a prolonged period. Risk factors for the development of PRIS include a critical illness such as sepsis, head trauma, use of vasopressors, and carbohydrate depletion (liver disease, starvation, or malnutrition). PRIS commonly presents as a high anion gap metabolic acidosis, with rhabdomyolysis, hyperkalemia, acute kidney injury, elevated liver enzymes, and decreased cardiac output. Because of the risk of PRIS, the duration of propofol infusion administration should not exceed 48 hours and the administered dose should not be higher than 4 mg/kg/hour.
This potential advantage of remimazolam over propofol will be offset by the increased expense of hours or days of remimazolam utilization in an ICU sedation situation. ICU sedation with fentanyl and older benzodiazepines such as Ativan will have the advantage of a lower cost.
In the hands of an anesthesiologist, propofol is an elegant and almost ideal intravenous sedative, with the advantages of rapid onset, rapid offset, inexpensive generic pricing, minimal cardiovascular/respiratory depression, and lack of nausea. Propofol administration does carry the risks of upper airway obstruction, hypoventilation, and low oxygen saturation, but when an anesthesiologist is present these risks are minimal.
If a healthcare organization doesn’t want to employ an anesthesiologist or a CRNA for a case which requires procedural sedation, then remimazolam may be an excellent sedative choice. Will gastroenterologists prefer to sedate patients with remimazolam plus fentanyl without an anesthesiologist? Or will they prefer to have an anesthesiologist present to administer propofol? Expect gastroenterologists to prefer the latter, because they are not only off-loading the task of sedating the patient, they are also off-loading the risks of managing the patient’s medical co-morbidities, which can be significant if a patient has lung disease, cardiac disease, morbid obesity, or obstructive sleep apnea.
The remimazolam story suggests one of my favorite anecdotes: A former Stanford Chairman of Anesthesiology and friend of mine who left the university in 2006 to become a pharmaceutical company executive, first at Novartis and then at AstraZeneca. Ten years ago, when I asked him what new anesthesia drugs were in the pipeline, he answered, “None, and there probably will be very few new ones. The drugs you have now are inexpensive generic drugs, and they work very well. The research and development costs to bring a new anesthetic drug to market are prohibitively expensive, and unless that new drug is markedly better, it will not push the inexpensive generic drugs out of use.”
Remimazolam will capture a very small market in the United States. Until remimazolam becomes an inexpensive generic drug, I see it as a medical white elephant rather than a wonderful anesthetic advance.

*
*
The most popular posts for laypeople on The Anesthesia Consultant include:
How Long Will It Take To Wake Up From General Anesthesia?
Why Did Take Me So Long To Wake From General Anesthesia?
Will I Have a Breathing Tube During Anesthesia?
What Are the Common Anesthesia Medications?
How Safe is Anesthesia in the 21st Century?
Will I Be Nauseated After General Anesthesia?
What Are the Anesthesia Risks For Children?
The most popular posts for anesthesia professionals on The Anesthesia Consultant include:
10 Trends for the Future of Anesthesia
Should You Cancel Anesthesia for a Potassium Level of 3.6?
12 Important Things to Know as You Near the End of Your Anesthesia Training
Should You Cancel Surgery For a Blood Pressure = 170/99?
Advice For Passing the Anesthesia Oral Board Exams
What Personal Characteristics are Necessary to Become a Successful Anesthesiologist?
READ ABOUT RICK NOVAK’S FICTION WRITING AT RICK NOVAK.COM.


Dr. Novak,
My name is Michael Brody. I’m also an anesthesiologist.
Though I agree with every point you made about remimazolam, I think you over-emphasized the actual cost of drug and minimized the the niche of use for non-anesthesia providers.
Per the cost: propofol is definitely cheaper; but the price point of Remimazolam is not astronomical. I think we all should be able to give it a try. There are many cases we do that would benefit with this new drug- TEE/cardioversion; neuro-interventional; GI cases in sick patients; ct/ir procedures ; etc. Though we, as anesthesiologists, are truly masters of propofol, and can give proper doses (and occasional jaw thrust) to get almost any patient through a procedure, it took many years in our hands to gain experience with it – using it in ways we initially never thought to do. In our OR: most of us now pre-op sedate patients in outpatient setting (sometimes inpatient if they are elderly) with propofol. That is not how propofol was initially described for use. Only through using it did we understand the nuances of the drug. And I feel with Remimazolam, we will figure out niches that this drug is much better and safer than propofol.
And saying cost should prevent us from trying is disingenuous and demonstrates the absurdity of financial responsibilities that seem to always have a double standard when anesthesia is involved.
Yes- we are doing more and more GI cases. And our department is still getting adequate reimbursement for the procedures (guessing $150-200 per case). But as Medicare (and then private insurances)continues to cap total reimbursement for GI cases, it will be a money loser to pay for anesthesiologist/CRNA – and an unnecessary labor burden on anesthesia departments when there already is a shortage of personnel. I feel eventually GI Docs with prefer to sedate ASA 1 and 2 patients and make more money. I think this will be a huge niche for this drug. Unfortunately: they frequently don’t really know how to dose drugs based on weights; along with interactions with other drugs patients are taking; other co-morbidities. In our hands, we can develop experience in how to dose; and when the time comes for non-anesthesia personnel to use drug, we will have valuable “practical” (non-pamphlet!!!) experience to guide them. Not sure if you remember, but versed was almost taken off the market when it came out because non-anesthesia providers were slamming 5 mg at a time because that is what they did with Valium- and it resulted in morbidities.
And per the scales of economy: we frequently run two rooms for one surgeon – yet we have entire surgical staff / anesthesia staff sitting on their hands for non-billable hours – just to save 15 minutes of turnover; in intervention rooms- how many times do proceduralist asks for “15 fr right curved catheter”….it’s opened – and then they say “oops ….I meant 13fr “……and hospital has to eat the $800 oops.
Bottom line: though I agree with most of what you said, if we followed this line of reasoning we would still be using isoflurane; pancuronium; etc….. I have not had the chance to use Remimazolam yet- but really want to try it. Our pharmacy refuses to put it on formulary until more safety and efficacy studies are done. Unfortunately with articles like yours, it lessons the chance of this drug to get into anyone’s hands to even do these studies. And I feel only in the hands of anesthesiologists can we truly decide if this drug is a medical white elephant.
Sincerely,
MIchael Brody, MD
Dr. Brody,
You raise several excellent points:
1. Cost shouldn’t be the only reason anesthesiologists make decisions. When sevoflurane came out, it was significantly more expensive than isoflurane, but our facilities purchased the drug, we used it, and discovered that indeed our patients woke up faster than they did with isoflurane, and our mask inductions went smoother with sevo than with halothane.
2. If gastroenterologists somehow, someday, actually lose income by employing anesthesiologists/CRNAs+ propofol, they may choose to jettison the anesthesia team and use remimazolam by themselves.
3. It is fun to try new medications, to learn new techniques, and to add to the number of arrows in our quiver by using a new anesthetic such as remimazolam. Many of us are still waiting since the June 2020 FDA approval to get that opportunity.
4. Running two operating rooms for one surgeon is a very costly strategy for hospitals and for anesthesia practices. The cost saving of $32.47 for one vial of propofol versus one vial of remimazolam is a trivial amount in comparison to the costs of keeping a surgeon happy by running two rooms for him or her.
Time will tell whether remimazolam finds its way into our tool chest. Thanks for contributing to the discussion!
Rick Novak
Dr. Novak, my name is Randy Ostroff. I am an anesthesiologist now practicing part time in Chicago. I also have had extensive involvement with the development of remimazolam in the US starting in 2011. I was not familiar with you or your blog until a colleague passed on your commentary regarding remimazolam. After reading it I felt compelled to respond so that you and your readers could be appropriately informed.
Your original contention is as follows:
“For use in procedural sedation, remimazolam will not replace Versed, but rather will aim to replace propofol.”
This is not entirely true. The commercialization strategy in the US initially was to replace Versed in combination with fentanyl for proceduralist driven sedation, primarily gastroenterologists and pulmonologists. As commercial partners changed from a GI focused to an anesthesia focused company as well as other factors that occurred, the strategy evolved such that it was subsequently decided to introduce remimazolam in the US primarily through the anesthesia community. However, this does not negate the strong business case that still exists for remimazolam to replace Versed for qualified proceduralist driven sedation of ASA 1-2 and some 3’s without an anesthesia provider. For instance, in a busy GI practice, the cost of remimazolam/fentanyl in place of Versed/fentanyl would be offset by the ability to perform 1-2 additional procedures in the same timeframe by alleviating the “choke point” or rate limiting step that occurs in recovery. Patient satisfaction is also high similarly to propofol as patients feel more normal and can resume activities sooner than after fentanyl/Versed. Presumably, the sicker and higher risk patients would still be done under the supervision of anesthesia providers, but for most patients remimazolam would allow the proceduralists to safely provide the same pharmacokinetic benefits as propofol without the additional hassle and cost incurred when anesthesia is involved.
The increased presence of the anesthesia specialty in the G.I. suite has frankly been driven primarily by the favorable economics. However, payors like Medicare and other third-party payers do not appreciate the additional expenses for anesthesia provided sedation which are often similar to or in excess of the proceduralist’s fees. Medicare has already pushed back on the gastroenterologists with carve outs from their fee for anesthesia since they had originally intended for their global fee to include both. There has been an ongoing controversy regarding the cost benefit ratio of having anesthesia providers sedating lower risk patients in the GI suites. An article published in JAMA in 2012 (Hangsheng Liu et al. JAMA.2012;307(11):1178-1184) identified a potential health system benefit of $1.1 billion if Medicare and other 3rd party payers did not have to cover separate anesthesia services for lower risk patients. That was based on 2003-2009 data with respect to colonoscopies, so the opportunity will only have increased since. Even a small percentage of that number easily justifies a few hundred million dollars in sales, does it not? Not to mention alleviating issues with arranging anesthesia coverage in GI offices or surgery centers. A secondary benefit would be a reduction or elimination of the dubious practice where gastroenterologists are employing anesthesia providers and increasing their own revenues by taking a cut of the anesthesia fees. Besides the questionable legalities, these practices only serve to further degenerate our speciality. As Dr Brody also correctly mentioned, you will see how quickly our toon changes when reimbursements decline for such coverage, but all of this will actually help to alleviate some of the projected shortages of anesthesia providers in the ORs where they are most needed. A hospital administrator may also see the advantages with regard to staffing if he can reduce the use of anesthesia services appropriately in G.I. suites, etc. and alleviate staffing issues in the OR, which is their highest revenue producer.
The real point is that remimazolam provides the combination of the safety profile of a benzodiazepine with the kinetics of propofol, which would seem to easily translate into multiple potential applications for anesthesia providers if you ignore cost for a moment.
So, where are the advantages for anesthesia providers to offset the cost? Here you are correct in that propofol would be a primary target, and have made the following statement:
“ Are these advantages important? Items 1 – 5 are discussed as follows:
Non-anesthesiologists can reverse the effects of remimazolam with flumazenil if they overdose a patient, but this advantage is less important for anesthesia professionals. Anesthesiologists can manage the airway of a patient over-sedated with a benzodiazepine without need to administer a reversal agent. I’ve never administered a dose of flumazenil in my entire career, nor have most of ”
Although I mostly agree, you neglected to consider the many situations where we are asked to provide sedation and do not have access readily to the airway. Some examples include in MRI or CT or other non-OR areas as well as procedures occurring on the head and neck under sedation. Are you aware that in the pivotal colonoscopy trial over 300 patients were sedated with remimazolam without the need for as much as a single jaw lift? Can you say the same about propofol and can you not see the utility?
You further stated:
“Propofol has cardiac and respiratory depression, but in most cases these effects are minimal. Per the PDR (Physician’s Digital Reference), patients with compromised myocardial function, intravascular volume depletion, or abnormally low vascular tone (e.g. septic patients) are more susceptible to hypotension. When an anesthesiologist is present these risks are routinely managed.”
I believe you have inappropriately minimized the hemodynamic effects of propofol at a time when there is increasing evidence that even modest degrees of hypotension for relatively brief periods may lead to detrimental outcomes. Certainly we’re well aware of the risks and consequences of hypotension in the “cardiac risk” patients.
As a result you have totally neglected to consider the possible uses and benefits of remimazolam as an induction agent for general anesthesia where the hypotension from a larger bolus of propofol is more frequent and profound. In fact, isn’t induction of anesthesia the original indication that was the introduction to the market of propofol or more specifically Diprivan in the US? MAC and ICU sedation indications followed later as would other and more expanded indications for remimazolam. Induction of GA seems a natural extension that you have somehow completely missed? At the very least it should supplant etomidate and other “cardiac” inductions. However, remimazolam could also potentially become the next gold standard in agents for the induction of GA, similarly to propofol which totally displaced and eliminated thiopental even prior to becoming generic.
Longer sedation cases will also be facilitated in the future by introducing infusions, which will make the comparisons to propofol easier. This application would be especially attractive for me as I perform MAC for some lengthy head and neck procedures that can be challenging with propofol. As Dr Brody also pointed out, once in our hands remimazolam users will find where it specifically has utility in their practice and that will determine the ultimate success or failure. I can easily think of several such potentially beneficial uses off the top of my head which apparently you did not consider such as cardioversions for example. I’m also hearing a number of admittedly anecdotal success stories from other clinicians regarding a variety of applications ranging from PNBs to TEE, etc. And as you can see, there are instances within an anesthesia practice that it will also potentially replace Versed.
Furthermore, as adept as we have become at the use of propofol, there are still a growing number of cases being reported concerning disasters with the use of propofol in obese and sleep apnea patients in the prone position. Do you not see utility there that perhaps justifies the expense?
I’m sure the list will only continue to grow, but not if we are not even given the chance to adequately evaluate the drug fueled by negative articles such as yours. The challenges are already great enough in the generic and Covid driven environment in which remimazolam was launched.
I have copied and pasted the remainder of your points below with my comments included in caps.
For a long operating room anesthesia case (e.g. of 8 – 10 hours duration), there is no clinically significant accumulation of propofol in the bloodstream. Propofol Infusion Syndrome (PRIS), which can be potentially fatal, is a risk with prolonged propofol sedation in the ICU (See ICU Sedation below).
AGREE WITH COMMENTS REGARDING PRIS
DISAGREE REGARDING ACCUMULATION ISSUES WITH PROLONGED PROPOFOL INFUSIONS. I HAVE PERSONALLY PERFORMED NUMEROUS 4-8 HOUR GA CASES WHERE PROPOFOL IS USED IN THE BACKGROUND AT ONLY 50-100 MCG/KG/HR FOR IT’S ANTIEMETIC EFFECTS. THE WAKE-UPS ARE DEFINITELY MORE VARIABLE AND SOMETIMES PROLONGED. ADDITIONALLY, MANY WILL RESUME SPONTANEOUS VENTILATION ONLY AT VERY HIGH CO2 LEVELS.
The burning sensation upon injecting propofol can be blunted by intravenous lidocaine. A 2016 meta-analysis showed that both lidocaine pretreatment and mixing lidocaine with the propofol were effective in reducing pain on propofol injection. In addition, a preanesthetic dose of Versed prevents a patient from remembering any burning sensation from a propofol injection that follows. AGREE, HOWEVER SOME PATIENTS STILL EXPERIENCE PAIN AND STILL RECALL THE DISCOMFORT DESPITE LIDOCAINE AND VERSED
The most important advantage of remimazolam is that non-anesthesiologists can safely administer remimazolam. Propofol administration requires an experienced clinician, e.g. either an anesthesiologist, a certified registered nurse anesthetist (CRNA), or an emergency medicine physician. Per the American Society of Anesthesiologists: “The practitioner administering propofol for sedation/anesthesia should, at a minimum, have the education and training to identify and manage the airway and cardiovascular changes which occur in a patient who enters a state of general anesthesia.” AGREE AS I ADDRESSED THIS EARLIER
HOWEVER, YOU NEGLECTED TO MENTION ISSUES WITH CONTAMINATION OF PROPOFOL, RESTRICTED OPEN TIMES, WASTE AND MANUFACTURING SHORTAGES.
The disadvantages of remimazolam compared to propofol include:
Expense. The cost of a 20 ml (200 mg) vial of propofol is $9.20. The cost of a 20 mg vial of powdered remimazolam is $41.67.
Remimazolam is sold as a powder and must be reconstituted into a liquid before it can be injected intravenously. THIS SEEMS TO BE A VERY MINOR IMPOSITION, LIKELY NO GREATER THAN THE NEED TO OPEN ANOTHER SYRINGE AND DRAW UP LIDOCAINE.
BTW-MIXING LIDOCAINE WITH PROPOFOL ALTERS THE DISTRIBUTION AND SIZE OF THE LIPID GLOBULES, WHICH THEORETICALLY CAN RESULT IN MULTIPLE MICRO-EMBOLI. I’M NOT CERTAIN IF THIS ISSUE WAS EVER RESOLVED? (PHYSIOCHEMICAL COMPATIBILITY OF PROPOFOL-LIDOCAINE MIXTURE. YOKO MASAKI ET AL. ANESTH ANALG 2003 DEC.)
Remimazolam is currently approved as an anesthesia drug in Japan and South Korea, for intensive care unit sedation in Belgium, but only for procedural sedation in the United States, China, and Europe. In total, there are four possible applications for remimazolam. Let’s examine the pros and cons of using remimazolam in these four applications:
Preoperative sedation. Since midazolam (Versed) was approved in 1982, a standard anesthesia practice has included a 2 mg dose of Versed prior to surgery to calm a patient’s anxiety. In the 1980s my anesthesia chairman at Stanford received a letter from a postoperative patient in which she complained of being awake and very anxious in the operating room prior to the anesthetic for her breast cancer surgery. Our chairman lectured to us, “Do you know many patients are nervous prior to their anesthesia and surgery? Every one of them. We have an excellent drug for relieving preoperative anxiety, and that drug is Versed. Use it! Give your patient a dose of Versed before they enter the operating room. There are few significant side effects of one dose of Versed. Use it!” Will remimazolam replace Versed for this application? No. There is no advantage of the new, shorter acting, more expensive remimazolam over Versed for preoperative sedation. AGREE, THIS WAS NEVER REALLY AN INTENDED USE. HOWEVER, MANY ANESTHESIA PROVIDERS ARE GOING BACK TO ELIMINATING VERSED PRE-SEDATION AS SOME PATIENTS DO EXPERIENCE A HANGOVER.
Sedation for short procedures. This is the FDA-approved application for remimazolam in the United States. An example procedure would be a colonoscopy. Will remimazolam be widely used for colonoscopies in the near future? No, I doubt it. The cost increase is the main disadvantage. See the typical drug acquisition costs for three alternative sedation recipes for colonoscopy below:
$18.40 for 400 mg of propofol; or
$5.17 for fentanyl+Versed ($4.35 dollars for 6 mg of Versed plus $0.82 for 200 micrograms of fentanyl); or
$41.67 for 20 mg of remimazolam.
The increased cost per case is $23.27 for remimazolam over propofol.
The increased cost per case is $36.50 for remimazolam over fentanyl+Versed.
If a busy endoscopy center does 100 colonoscopies cases per week, the cost increase is $2327 per week for remimazolam over propofol, or $3650 per week for remimazolam over fentanyl+Versed. These are a prohibitive cost increases with no clear added benefits. The only way remimazolam could result in cheaper sedation costs would be if a healthcare system was looking to eliminating the cost of paying for an anesthesia provider for these procedures.
The pairing of remimazolam+gastroenterologist sedation rather than propofol+anesthesiologist sedation could afford significant cost savings for a healthcare system.
I WOULD REFER YOU AND YOUR READERS BACK TO THE JAMA AND NUMEROUS OTHER ARTICLES REGARDING THE COSTS OF ANESTHESIA PROVIDED SEDATION FOR COLONOSCOPY AS WE’LL AS MY PREVIOUS COMMENTS.
THE ECONOMICS AND PROJECTED ANESTHESIA PROVIDER SHORTAGES IN THE FUTURE WILL LIKELY DRIVE MORE SEDATION PERFORMED BY PROCEDURALISTS. WE ARE SEEN AS THE EXPERTS AND GUARDIANS IN THIS AREA, AND WILL BE THE ONES THEY LOOK TO FOR GUIDANCE. IT WOULD THEREFORE SEEM PRUDENT FOR US TO HAVE ACQUIRED AS MUCH KNOWLEDGE AND EXPERIENCE WITH A NEW DRUG AS POSSIBLE. BETTER TO BE PARTICIPANTS AND PROVIDE OUR COLLEAGUES WITH GUIDANCE AND A SAFER BULLET THAN TO BE OBSTRUCTIONISTS PROTECTING OUR OWN FINANCIAL INTERESTS.
3. Total intravenous anesthesia (TIVA). TIVA could include a continuous infusion of the ultra-short-acting narcotic remifentanil plus a continuous infusion of the ultra-short-acting remimazolam. An alleged advantage of this technique could be the fast offset time of these two TIVA anesthetic agents. I doubt this technique will gain market share. It’s far easier to turn on the knob of a sevoflurane vaporizer than to load and manage two TIVA-syringe pumps. As well, the added expense of a prolonged infusion of remimazolam will be prohibitive. AGREE THAT TIVA HAS NOT BEEN WIDELY ACCEPTED IN THE US. HOWEVER, THERE ARE CLINICAL SITUATIONS WHEN INHALED AGENTS ARE STILL AVOIDED SUCH AS MH AND SSEP MONITORING. SOME OF THESE PATIENTS WILL NOT TOLERATE THE THE HEMODYNAMIC CONSEQUENCES OF PROPOFOL AND IT IS NOT CURRENTLY KNOWN WHETHER THE USE OF PRESSERS ALSO COUNTERACTS THE NEGATIVE OUTCOMES FROM HYPOTENSION.
FOR INSTANCE, HAVE YOU TRIED SEDATING AN ELDERLY PATIENT FOR A FACE LIFT WITH PROPOFOL AFTER THEY HAVE RECEIVED 0.2 MG OF CLONIDINE?
ICU sedation. Remimazolam has the advantage of ongoing first-degree elimination, meaning that no matter how long the drug is infused, it will always have reliable elimination by esterase and will not accumulate in the plasma. Prolonged ICU sedation with propofol can lead to the Propofol Infusion Syndrome (PRIS). PRIS occurs predominantly in patients receiving high doses of propofol for a prolonged period. Risk factors for the development of PRIS include a critical illness such as sepsis, head trauma, use of vasopressors, and carbohydrate depletion (liver disease, starvation, or malnutrition). PRIS commonly presents as a high anion gap metabolic acidosis, with rhabdomyolysis, hyperkalemia, acute kidney injury, elevated liver enzymes, and decreased cardiac output. Because of the risk of PRIS, the duration of propofol infusion administration should not exceed 48 hours and the administered dose should not be higher than 4 mg/kg/hour.
This potential advantage of remimazolam over propofol will be offset by the increased expense of hours or days of remimazolam utilization in an ICU sedation situation. ICU sedation with fentanyl and older benzodiazepines such as Ativan will have the advantage of a lower cost.
TO SOME EXTENT I AGREE, BUT IT SEEMS LIKE YOUR ONLY UNDERSTANDING OF COST IS BASED SOLELY ON ACQUISITION COSTS. THIS IS A VERY MYOPIC VIEW. THE OLDER DRUGS YOU’VE MENTIONED ARE CHEAP, BUT ANY SAVINGS IN ACQUISITION COSTS ARE QUICKLY LOST IN INCREASED VENTILATOR AND ICU TIMES. ALSO CONSIDER, WHAT ARE THE COSTS IN DOLLARS, NOT TO MENTION THE HUMAN ELEMENT, WHEN A PATIENT DOES MANIFEST PRIS?
BUT LET’S LOOK AT ANOTHER ICU SEDATIVE DRUG, PRECEDEX, AS AN EXAMPLE . PRECEDEX FACED THE SAME HURDLES UPON ITS INTRODUCTION INTO ICU. DESPITE A PROPRIETARY PRICE IN A GENERIC ENVIRONMENT AND DUE TO ITS UNIQUE PROPERTIES PRECEDEX SUCCESSFULLY CAPTURED A SIGNIFICANT PERCENTAGE OF USE IN THE ICU. SUBSEQUENTLY ADDITIONAL INDICATIONS WERE PURSUED, WHICH RESULTED IN > $300 MILLION IN ANNUAL SALES. I DON’T THINK ANYONE WOULD DESCRIBE THAT AS A WHITE ELEPHANT. COMPARING THE PHARMACOLOGY BETWEEN REMIMAZOLAM AND DEXMEDITOMIDINE, IT WOULD SEEM PRETTY OBVIOUS THAT REMIMAZOLAM HAS A FAR BROADER POTENTIAL.
ONCE AGAIN, IT’S SOMEWHAT BAFFLING HOW YOU COULD WRITE THIS PIECE AND COMPLETELY IGNORE THE USE AS AN INDUCTION AGENT FOR GA. I CAN ONLY CONCLUDE THAT EITHER YOU DID NOT ADEQUATELY DO YOUR HOMEWORK OR THAT YOU INTENTIONALLY OMITTED AS A RESULT OF SOME INTENTIONAL BIAS?
Admittedly, remimazolam likely does not have the anti-emetic affects of propofol. That is one of the few real disadvantages if you remove price from the equation, which seems to be your primary concern and criticism. This type of thinking only serves to further denigrate our specialty as we are leveraged to cut relatively insignificant amounts of cost versus what is tolerated by our surgical colleagues despite that we are only a small percentage of the total. Shouldn’t you instead be embracing new entries into a market that has largely been abandoned by the larger Pharma companies. This is a chance for us to champion our value, which lies in the evaluation of such new entities as well as identifying appropriate uses that potentially improve quality and/or efficiency? Certainly cost should be a consideration, but not the primary or rate limiting one.
Nobody is realistically expecting a proprietary drug to enter a generic market and dominate or completely replace propofol or Versed. However, there will be multiple applications dependent on specific practices etc. that will undoubtedly produce scenarios where the cost will be justified. Plus there are numerous practices where cost is not such a big driver. Like Diprivan and Ultane, remimazolam will ultimately become generic and you can have your cake and eat it too! Until then, understand that innovation from Pharma has been a huge contributor to the advances in the practice and safety of anesthesia. Innovation does not come without risk and there has to be a potential reward.
Remimazolam was launched at a difficult time during a pandemic. Why not let the marketplace determine the overall value and not prejudge?
In your response to Dr. Brody you mentioned SEVOFLURANE as an example, ironically another drug with which I have considerable experience and familiarity in regards to the development and commercialization. You specifically stated that upon using sevo you confirmed the superior wake up etc. which presumably justified the higher price. I can only suggest that you give remimazolam the same consideration and to reserve judgment until then. I firmly believe you will also have similar revelations once you gain some personal experience.
Sevoflurane also serves as an example of the trends in anesthesia drug development that have produced faster onset, shorter acting and more predictable drugs with less hemodynamic consequences based on reduced organ dependent metabolism and elimination. Rocuronium is another example, successfully replacing vecuronium. Remimazolam reflects the current peak of this same evolutionary trend with respect to benzodiazepines.
The field of anesthesia has experienced tremendous advances with respect to the pharmacology of the drugs we use now as compared to even when I trained 40 years ago. The benefit of these advances to society and healthcare in general are tremendous, but if we except the current generic world as good enough because we can accommodate it’s deficiencies then we will stifle any future innovation and our specialty will not advance.
I believe Dr. Brody expressed it in a way that I could not have said much better myself. To paraphrase, with your type of thinking we would still be using thiopental, isoflurane and pancuronium or at best vecuronium.
Thank you for your consideration of my response. I apologize for the lengthiness, but felt it was important to fully inform you and your readers, so I hope you will post in its entirety.
I look forward to your response.
Dr. Ostroff,
Thank you for your comprehensive and lengthy essay. These are my responses:
1. Remimazolam replacing propofol for endoscopy. I agree with you that employing anesthesiologists for propofol sedation for low-risk colonoscopy is not cost-effective healthcare. Like you, I can envision a model where gastroenterologists sedate ASA 1 and ASA 2 patients with remimazolam, rather than employing anesthesiology professionals to administer propofol. I still see this as the biggest potential market for remimazolam. I agree the current expense for GI sedation using anesthesia provider + propofol are high, and that GI doctor + remimazolam will save the cost of paying for an anesthesia provider + propofol, if there is some financial incentive for the healthcare system to save this cost.
2. Remimazolam replacing Versed + fentanyl for endoscopy. You cite this strategy because of the fast discharge to home time with remimazolam, which will eliminate the “choke point” of multiple patients building up in the PACU after receiving Versed + fentanyl, saving money for the healthcare system. If gastroenterologists want to eliminate their dependence on anesthesiologists, one can envision a model where gastroenterologists sedate ASA 1 and ASA 2 patients with remimazolam, rather than employing Versed + fentanyl. (Reference: A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy https://pubmed.ncbi.nlm.nih.gov/29723512/ . This strategy may entice GI doctors who still use Versed + fentanyl to switch to remimazolam, despite the higher acquisition costs.
3. Remimazolam for induction of general anesthesia. I don’t see remimazolam ever having a significant market as an induction agent for routine general anesthetics. I’ve personally administered propofol as the induction agent for general anesthesia tens of thousands of times, and it’s a remarkably safe drug. For the rare patient with significant cardiac dysfunction or unstable hemodynamics, etomidate is a preferred alternative which is also an inexpensive generic drug. I can foresee a use for remimazolam as an induction agent for a patient with an expected difficult airway. If the anesthesia provider runs into a dire cannot intubate-cannot ventilate (CICV) situation after induction with remimazolam and rocuronium, he or she can reverse both drugs with flumazenil and sugammadex, and wake up the patient safely. This could be an important use for remimazolam.
4. Propofol infusions causing inconsistent wakeups after long (4 to 8 hour) general anesthetics. This has not been my experience, nor am I aware of any support for this contention in the medical literature.
5. Anesthesiologists becoming expert with remimazolam so we can advise GI doctors, pulmonologists, and other doctors administering procedural sedation. I support your reasoning on this point. I would welcome the opportunity to become expert with remimazolam for procedural sedation, despite the economics, so that I would have one more tool in my toolbox. If remimazolam is similar to propofol for procedural sedation, but more costly, I still don’t see hospitals and healthcare systems advocating for its routine use. And if remimazolam is similar to propofol for procedural sedation, I cannot imagine anesthesia companies advising GI doctors, pulmonologists, and other doctors to decrease using anesthesiologists for these procedures, unless the anesthesia company is interested in losing business and/or losing the responsibility for covering procedural sedation. Most anesthesia companies are looking to expand their bottom line by expanding their workload of insured cases. For low-reimbursement Medicare procedural sedation cases, for which anesthesiologists are being paid perhaps 1/5th of the commercial rate, it’s likely an anesthesia company would prefer that Medicare procedural sedation be done by GI doctors, pulmonologists, and other doctors using remimazolam, rather than by their anesthesiologists.
6. Why has remimazolam been FDA-approved since June 2020, twenty-two months ago, yet there has been minimal market penetration of this drug? This is a key question, and the reason I chose to write the column. If remimazolam is to become an important anesthetic/sedation drug in the United States, we’re still waiting for this to be true. As you said, “Remimazolam was launched at a difficult time during a pandemic. Why not let the marketplace determine the overall value and not prejudge?” These are wise words. My intention was not to prejudge, but to opine on the state of remimazolam as of March 2022. The marketplace will indeed determine the overall value of this new drug. As of this time, remimazolam is not available on the list of pharmacy drugs at Stanford University Hospital.
Dr. Novak,
Thank you for publishing my entire post and for your response. However, I still think you’re missing the point judging by many of your responses.
Without getting into a prolonged back and forth, let me just briefly make a few more points.
I believe your agreement in point 1 is already an acknowledgement of the business case that was used as the initial motivation to develop remimazolam. However, it will really be the economics and the 3rd party payers that will drive the decreases in our presence during procedural sedation, not the company. Once Medicare sets the bar, generally the others will follow. As the reimbursements become less favorable for both you and the proceduralists to continue as is, and additionally become more favorable for the gastroenterologists to provide their own sedation, you can predict what will happen. In that scenario you’ll happily be touting better ways to appropriately utilize anesthesia services. But I would hope that these decisions would be based on risk related clinical factors, and not on reimbursement as you seem to have suggested. The suggestion that the provision of anesthesia services would be based on Medicare or any payer seems questionable.
Again, I believe you are minimizing the issues with respect to propofol inductions and hemodynamics. I don’t think that cardiac and other patients where we are concerned about hypotension are “rare.” If propofol is such a “safe” drug, why is its label restricted to persons with a higher level of training in anesthesia? It’s not only the hemodynamics. I have also noted that the 2 drugs have very different effects on the airway. In your CICV scenario, it is altogether possible the cannot ventilate part could entirely be avoided by using a remimazolam induction with spontaneous ventilation. This would also be applicable to the more common suspected difficult airway, etc. Yes, there are alternatives like etomidate as you mentioned. Etomidate is not without its issues and there have previously been shortages that have driven the price up at times even higher than Byfavo. And you still cannot reverse etomidate.
You seem to want to look at this with the mentality of a hospital pharmacist in that remimazolam would totally replace either Versed or propofol. That’s not what I’m saying. What I am saying is that remimazolam will provide a viable alternative for select patients and/or procedures. We have identified many of them, but only through ongoing research and clinical practice will we each decide its ultimate utility and place in our armamentarium and practices. Which brings me to my final point, your “stated” reason for writing this blog. For your information, although approved in July (not June), 2020, due to Covid, the drug was not actually launched (sold) until early 2021, slightly more than 1 year ago. That was with a relatively small number of sales reps that had to be trained virtually and were largely restricted from any face to face interactions with clinicians. The focus and distractions from Covid make any conclusions regarding remimazolam sales very difficult. I would ask you, what is the basis of your statement regarding “minimal market penetration?” Do you have those figures? Are you aware of how they compare to projections? Do you have data regarding formulary approval? More importantly, do you have any previous experience or basis for comparison, pandemic aside? I gave you the example of Precedex in my initial response. When the spinoff of Hospira occurred from Abbott, the sales of Precedex were so low after more than three years post approval that Abbott had no qualms regarding leaving it with Hospira. However, sales of Precedex subsequently took off such that in strategic meetings at Abbott there were serious discussions about buying Precedex back to bolster their anesthesia franchise when sevoflurane became generic. The race is not determined in the first lap.
In conclusion, shouldn’t a prestigious institution such as Stanford be a leader? I would think that you’d embrace a new entity like remimazolam, merely for the teaching and potential research aspects? How many companies are still investing in drug development in our specialty, and when was the last novel drug introduced? Perhaps your efforts would be better served in that direction?
Perhaps I could also suggest the following. Instead of complaining about the price, and publishing your opinion in a non-peer reviewed format, you could propose an investigator initiated clinical trial from the company? That may provide you with an opportunity to gain familiarity with the drug while answering a specific scientific question. Remimazolam is still in its infancy and there are numerous unknown scientific questions.
(Note-One quick aside you also neglected, pediatric ICU sedation. This is where PRIS is more of an issue and there is no real ideal alternative)
BTW-if we keep this up, we will eventually identify enough uses that even you might become an advocate… Lol
Hi Dr Novak,
Read with interest your article regarding this new amnesties medication remimazolam.
I used to be a big proponent of using an amnestic, analgesic and hypnotic medication to achieve balanced anesthesia in Endoscopy procedures but over the two decades I have been practicing anesthesia I have stopped using any amnestic or analgesic medication and have relied entirely on the hypnotic-sedative effects of propofol.
I have never had a patient complain of awareness or pain as most of these procedures have only minimal discomfort.
Using any other medication besides propofol doesn’t add anything to the anesthesia care on the contrary prolongs PACU stay in ASA 3 or 4 cases which is majority of my practice.
We don’t need remimazolam if we titrate or current medications an excellent anesthesia care can be achieved for endoscopic procedures.
Dheeraj,
I agree. We used to administer Versed along with propofol for endoscopy sedation, but for years now have converted to using propofol alone. The gastroenterologists did not like the Versed side effect of the patient being amnestic in the PACU after the procedure–it interfered with the patient remembering the gastroenterologist’s explanation of the results of the procedure. From the anesthesia perspective, the omission of Versed made no difference in intraoperative awareness.
Dr. Nagpal,
My comments (and I believe Dr. Ostroff comments) were geared towards replacing propofol with Remimazolam; not in addition to Propofol.
In the early studies done, it already shows faster wake up than propofol; less airway obstructions; and less negative viral sign variability. I feel that there could be true potential benefits of using this drug in ASA 3 and 4 patients; and allow proceduralists to use it ( ASA 1 and 2) when anesthesia not available or cost effective.
A quick update. Although remimazolam may not be on formulary at Stanford yet, they are currently enrolling patients into a pivotal pediatric study.
Also, Byfavo is currently on formulary at Mayo Clinic, John Hopkins, MUSC and other prestigious institutions.
Regarding your assertions concerning the safety of sedation in our hands (MAC) with the currently available agents Versed & propofol, I would refer you to a recent look at the ASS closed claims database:
https://www.expertinstitute.com/resources/insights/most-common-anesthesia-injuries-insights-from-malpractice-insurance-claims/
“In the 1980s, MAC claims represented 2% of claims—this figure has risen to 10% in 2000 and after. Although the use of airway instrumentation is minimal at most, and the amount of anesthetic is usually much less in a MAC technique. Death was more common with MAC techniques than in general or regional anesthesia. One possible explanation is that MAC may provide a false sense of safety to anesthesia providers, thereby, increasing the possibility of negative outcomes.”
“During MAC anesthesia, the airway is not protected. However, potent medications are given to keep the patient comfortable during the procedures. It is not surprising then that medication overdose by anesthesiologists was the most common mechanism of injury. It accounts for 21% of MAC claims. Over half of over sedation cases involved combinations of propofol and benzodiazepines or opioids (such as versed and fentanyl).”
Based on this information, seems like there is still considerable room for improvement even when anesthesia providers are involved in the sedation. I currently don’t have the data to claim that remimazolam is the answer, however, based on the pharmacological properties of remimazolam (short duration, non-accumulation, lack of active metabolite reversibility), it would appear to have the potential to prevent many of these occurrences.
As the use of sedation (CS and MAC) continues to increase, it only becomes more imperative for us to explore even incrementally safer options.